When light travels through air or space, it moves unimaginably fast—circling the Earth about seven...
Photon Transport Simulations with Monte Carlo: A Practical Guide for Medical Device Designers
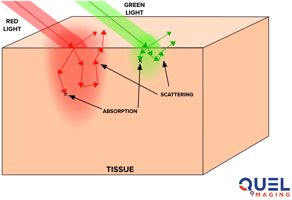
Designing an optical sensor for medical devices often feels like educated guesswork. You know your LED needs to illuminate tissue and your photodetector needs to capture the light that bounces back—but what's actually happening to the photons in tissue?
Photons travel through millimeters of skin, fat, mucosa, or muscle that can be densely seeded with vasculature, lymphatics, and connective tissue. How deep do your photons penetrate tissue? What percentage are absorbed versus scattered? Will moving your detector 2mm dramatically improve your signal, or barely make a difference?
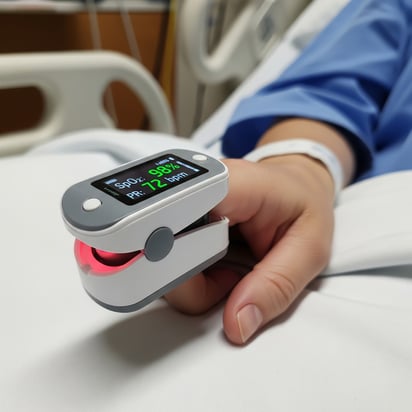
Without visibility into these subsurface photon interactions, engineering teams are left building prototype after prototype, tweaking designs, swapping LEDs or detectors, and hoping empirical testing will eventually reveal an optimal design. Testing dozens of design variables in humans and animal models can delay your product launch indefinitely if the variability is not managed. Simulating photon transport in tissue becomes a critical tool in the early design stages of device development.
Monte Carlo photon transport simulations are a powerful design tool for optical sensing. These models generate and follow millions of individual photons as they travel through tissue, scatter off cellular structures, get absorbed by chromophores like hemoglobin and melanin, and occasionally hit your photodetector. Instead of wondering what's happening beneath the skin, you can see it: detailed maps of photon migration, quantitative predictions of detected signal strength, and clear insights into how design parameters influence optical performance.
Simulations help establish critical product specs early in development, such as:
- targeted light fluence and source optical power
- illumination wavelength and spectral bandwidth
- illumination spatial pattern
- detector sensitivity
- detector positioning
- and much more.
Models and simulations can be extended to prototype signal processing workflows before ever turning on an LED. Importantly, they can help you capture the real world variability in user tissue variability to capture performance in different skin tones, cardiovascular conditions, and body compositions. They can save time and resources on mission critical efforts to get effective products to market.
Whether you are building a pulse oximeter, heart rate monitor, or phototherapy devices, monte carlo simulations help your team:
- Set technical specification early
- Design quickly
- Prototype confidently
- Iterate seamlessly
- Bring working prototypes to live testing scenarios with fewer surprises
At QUEL, we develop and use Monte Carlo simulations constantly to develop and check our tissue phantom designs - we have even developed our own implementation for simulating fluorescence. Our team helps medical and consumer product teams get the most out of Monte Carlo simulations to establish product specifications and test methods to bring their products to market efficiently. With decades of combined experience, we understand how to get the most out of simulations for product development.
Here, we will explain how these simulations work, what information is needed to get the most out of your simulations, and some of the tools we use to make that happen.
What is a Monte Carlo Photon Transport Simulation?
Generally, Monte Carlo simulations are used to run many individual scenarios with defined event probabilities.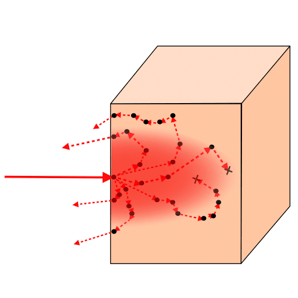
For photon transport, we use Monte Carlo simulations to trace photon trajectories through tissue with defined absorption, scattering, and directionality behaviors. We trace millions or billions of photons for a given model. From this, we learn where and how much light gets absorbed and scattered in tissue. These models are powerful for screening source and detector placements, refining refractive optical systems design, and varying tissue composition to determine the best specifications for a target application.
With this data, we can accurately predict where light goes in tissue, how much light hits the detector, and estimate tissue depth sensing capabilities based on tissue physiology. This helps set illumination power and wavelength, detector sensitivity, dynamic range, and signal processing specifications needed to design and prototype your device.
To get started, we need a physical model of a light source to seed photons and our representative tissue model to interrogate.
Light Source Definition
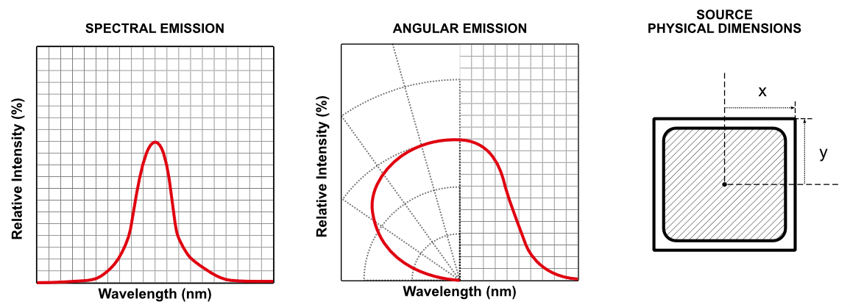
The key parameters to define for a light source ahead of simulations are:
- Spectral profile (i.e., illumination wavelength)
- Angular Emission Profile (direction)
- Physical size of the emitting source
Absolute power levels from a source can be applied retroactively to the analysis, since the simulation provides relative light distributions that scale linearly with incident light power.
Tissue Model Definition
This is where things get tricky. Tissue is highly variable within and between users. Modeling tissue requires knowledges of the tissue's optical properties and physical structure. For our models, we will combine direct measurements (see our spectrophotometry and time-of-flight spectroscopy posts) and published literature on tissues of interest. Ultimately, we need the following information for our material model: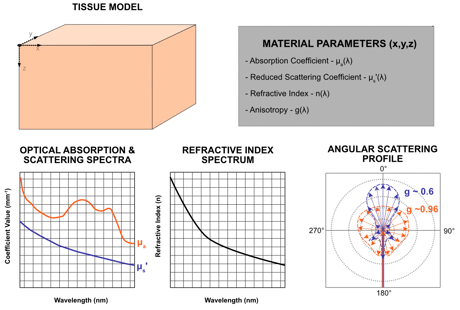
- Physical dimensions of tissue structure and substructure.
- Absorption Coefficient (µa) - defines the probability of photon absorption in a material over a given distance traveled.
- Reduced Scattering Coefficient (µs’) - defines the probability of photon scattering in a material over a given distance traveled.
- Refractive Index (n) - influences scattering probability and light direction through a given location
- Anisotropy (g) - defines the directional changes photons could have through a given location.
We define a 3-dimensional object (x,y,z) where each voxel or mesh node is assigned optical properties: µa[𝛌], µs’[𝛌], n[𝛌], and g[𝛌] parameters that represent how a photon (with defined 𝛌 and direction) interact when traversing a given voxel.
Simple tissue models of homogenous material are simple to define, but may miss some nuance in the underlying physiology being measured by your device.
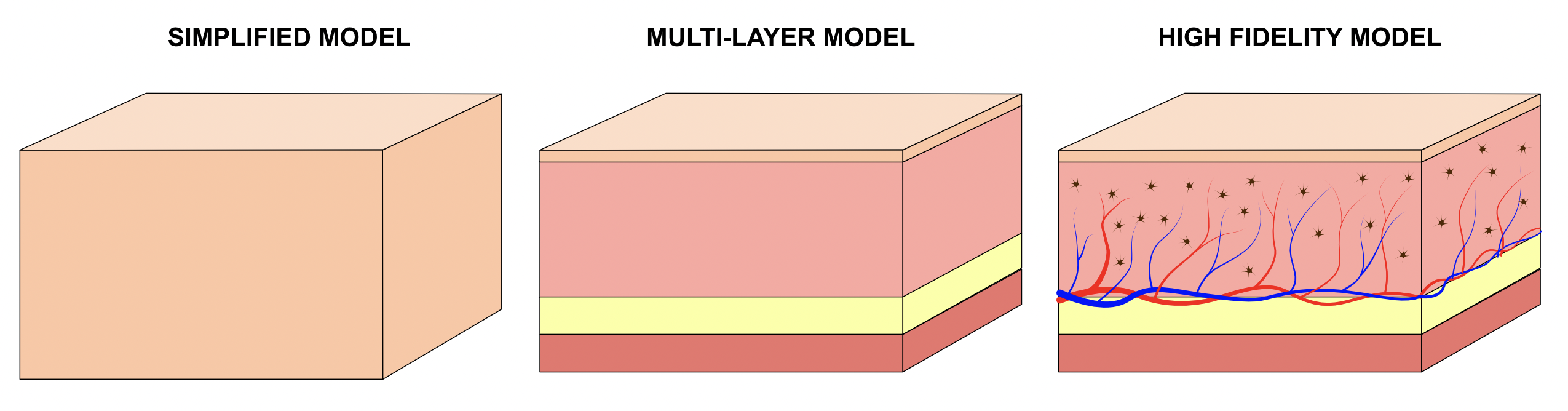
Higher fidelity models might include different layers of tissue substructure, each with their defined optical properties. This can be more realistic than simplified models in terms of understanding light transport, but may misrepresent any fine changes in physiological changes in target tissue.
The highest fidelity models account for sub-diffuse regime tissue architecture to include vascular, lymphatic, cellular, and oxygenation features in the tissue. This provides an accurate estimation of physiological readout at the expense of simulation size, speed, complexity, and variability. Practically, model fidelity is generally restricted to the relevant size scales of a particular application.
Deciding on tissue model fidelity is an application-specific question. Considering what information you need from your device and how model fidelity will influence those measurements will inform what approach makes sense for your needs.
A Photon’s Journey
With our source, and tissue models defined, we can follow a photon through its journey, starting at the illumination source.
The light’s wavelength and direction is probabilistically assigned based on supplier specifications for our light source. We launch the photon through space until it finds an interface with our tissue model.
With the wavelength and incidence angle known, we can "roll the dice" on whether the photon will be absorbed, scattered, or transmitted.
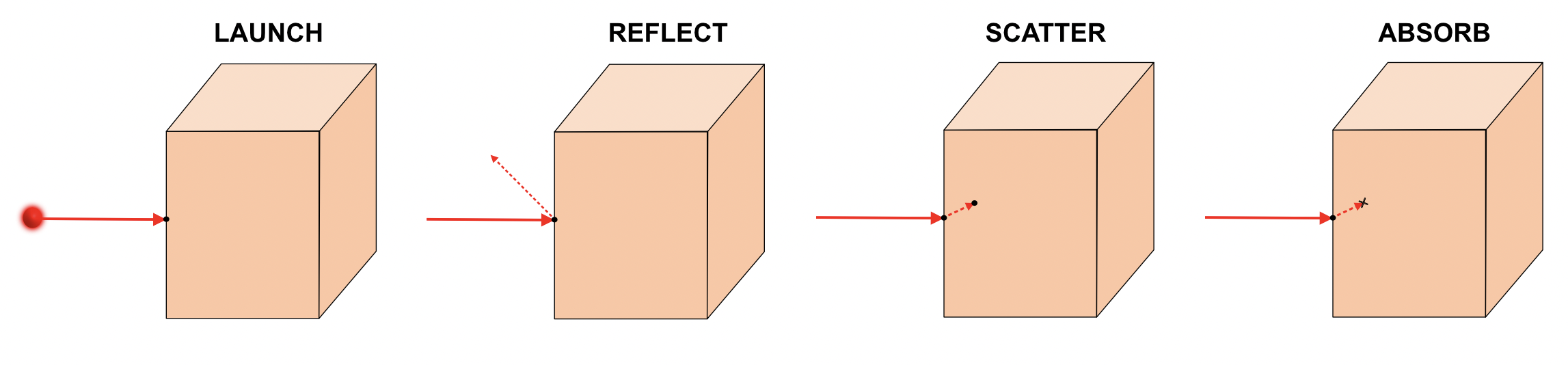
The absorption, scattering, and refractive index parameters define these probabilities. And the anisotropy defines where the photon will head next. With these properties determined, we propagate or terminate the photon to it’s next voxel location.
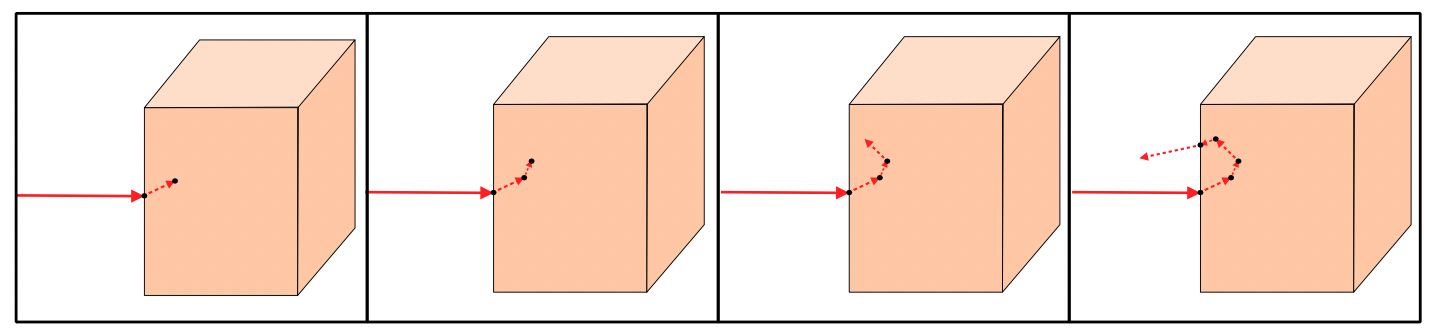
 This process is repeated until the photon exits the tissue model or gets absorbed. We store the individual photon path information and it’s terminal event, then we do it all over again - launching a new photon from our source.
This process is repeated until the photon exits the tissue model or gets absorbed. We store the individual photon path information and it’s terminal event, then we do it all over again - launching a new photon from our source.
As we accumulate simulated photons, the simulation maps the relative fluence of light absorbed or transmitted at any given voxel of the tissue model. With millions of photons simulated, we have a scalable model of photon transport in a given block of tissue.
Making Use of Simulation Data
From our simulations, we get a probabilistic representation of where photons end up in our tissue model. We can use this to screen detector specifications, detector physical placement, and extrapolate how much light is needed to produce optimal photosignals for our device.
We can also re-run these simulations while varying the relative source-detector positions. With photon paths known, we can calculate how much of our detected light is superficially versus deeply transiting through our tissue. Depending on your application, you can adjust detector positions, detector specifications, illumination wavelengths, and illumination patterns to optimize the nature of your detected signals. Perhaps a different illumination wavelengths gets more deeply transited photons to your detector, or a larger active area at a longer separation distance biases your detected signal towards deep-transiting photons. These are all application-specific considerations to weigh in designing your device.
Having these specifications in place takes a lot of the guesswork out from your technical team in selecting components, and prototyping your devices. This lets projects proceed quickly, confidently, and more efficiently.
Accounting for Tissue Variability and Physiological Dynamics
From a physics standpoint, tissue is messy. Variability’s impact on your device’s performance could mean the difference between a game-changing and a lackluster product launch.
 Your end users are all physiologically unique. Models and phantoms give us some control over tissue optical properties that we can account for in device design. Varying material properties and structure in our tissue models can help to capture things like:
Your end users are all physiologically unique. Models and phantoms give us some control over tissue optical properties that we can account for in device design. Varying material properties and structure in our tissue models can help to capture things like:
- Varying skin pigmentation
- Tissue oxygen saturation or perfusion status
- Sensing location on the body
- Adiposity
- Cardiovascular conditions
Each of these things may critically impact your device's performance and user experience - whether intentional or not. Monte Carlo photon transport simulations let you computationally benchmark your device across a broad base of real-world scenarios to make sure your device is safe, effective, and resilient in the hands of your diverse user base. Capturing these variables by systematically adjusting tissue model layer geometry, absorption, and scattering properties can help you gauge whether your device will perform across different critical user variables before testing in live subjects.
Simulating user variability in-silico can reveal design limitations before animal or human model testing. For example, if the detector-source offset prevents accurate measurements in darker skin toned patients or limits the dynamic range in higher adiposity users, simulations let you capture and adjust your design this before live experiments. Surprises like this appearing late in product development can massively delay a product launch. Simulations offer a way to get ahead of these surprises and help your team hit their development goals.
Extending Monte Carlo Simulations to Technical Device Design
The information afforded by photon transport simulations has logical extensions to other areas of technical device development.
Mechanical and Industrial Design
The optical specifications for your device inform the parts and packaging of your final product. Mechanical designers need this information to make high quality and performant product designs with the needed components. The technical specifications of these components can be established through Monte Carlo simulations by help screen for an optimal physical arrangement of optical components (e.g., illumination sources, refractive elements, detectors) to get the useful and repeatable device performance.
Signal Processing Development
 Knowing how much light is hitting your detectors gives your signal processing team some number to work with when choosing the right detector architecture, noise characteristics, dynamic ranges, and algorithmic approaches to captured photosignal. Providing software, firmware engineering teams with expected detector fluence ranges help ensure your device will do what it needs to do.
Knowing how much light is hitting your detectors gives your signal processing team some number to work with when choosing the right detector architecture, noise characteristics, dynamic ranges, and algorithmic approaches to captured photosignal. Providing software, firmware engineering teams with expected detector fluence ranges help ensure your device will do what it needs to do.
Embedded Systems Development
Understanding the power, timing, and processing systems needed in your device are influenced heavily by source optical power, the detector architecture, and temporal bandwidth of embedded systems, microprocessing power consumption, battery capacity, and much more. Monte Carlo simulations help get embedded systems professionals the information they need to design performant devices that deliver on customer expectations.
Tools for Monte Carlo Photon Transport Simulations
 At QUEL Imaging, we are big proponents of open-source computation. Our photon transport simulations primarily occur on the MCX platform - maintained by Qianqiang Feng's Lab at Northeastern University. The MCX software package is designed around GPU parallelization, which makes running simulations orders of magnitude faster than with CPU simulations. Further, extensions for fluorescence, Raman scattering, and time-series simulation capabilities are available with more to come.
At QUEL Imaging, we are big proponents of open-source computation. Our photon transport simulations primarily occur on the MCX platform - maintained by Qianqiang Feng's Lab at Northeastern University. The MCX software package is designed around GPU parallelization, which makes running simulations orders of magnitude faster than with CPU simulations. Further, extensions for fluorescence, Raman scattering, and time-series simulation capabilities are available with more to come.
Several commercial packages exist for photon transport modeling. Ansys (Zemax Nonsequential Mode, Speos), Synopsis/Keysight (LightTools, ImSym), Lambda Research (TracePro), and Photon Engineering (FRED) are some of the more popular offerings. At QUEL, we use TracePro to cross-validate our MCX simulations but do the bulk of our work in MCX.
All of these tools - whether open-source or commercial - have steep learning curves to make the most of them. Despite that, they offer a powerful toolset to derisk and constrain your device development for a successful product launch. Tissue optics expertise goes a long way in making effective use of these tools.
Summary
Photon transport simulations are a powerful tool to specify and derisk optical sensing device development efforts. Scoping technical requirements and design parameters before purchasing components and building prototypes help prevent costly surprises from delaying product launches. While the concepts of photon transport simulations are relatively simple, the implementation and analysis can be complex. In the hands of a talented technical team, the insights offered by these simulations can help set your development team up for success on your next launch.
At QUEL Imaging, we bring decades of combined expertise in tissue optics and imaging systems to support your product development efforts with world class tissue phantom production and optical characterization infrastructure. Learn more about how our team can help get your next product launched on schedule by reaching out today.