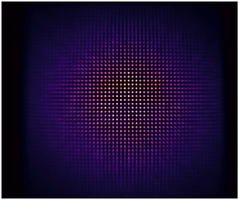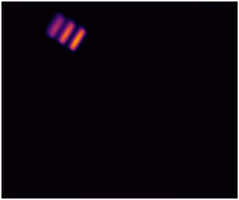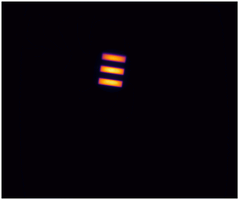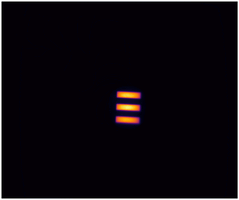
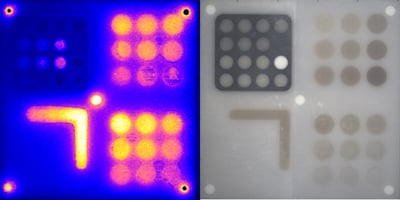
Imaging performance can be assessed in many ways, but two of the most critical aspects are how...
FDA issues draft guidance on optical imaging drugs
The FDA recently released draft guidance for optical imaging drugs, including an appendix dedicated to device characterization. These recommendations lay out key criteria that developers should be prepared to address in any 510(k) submission involving imaging systems.
At QUEL Imaging, we’ve publicly submitted comments on this draft guidance. While it’s a step forward, critical aspects of how tissue imaging systems should be characterized — and how different systems can be meaningfully compared — remain largely undefined in the medical device landscape. We believe that faster clinical adoption of optical technologies starts with clear, standardized methods for assessing device performance.
Until more definitive guidance is finalized, here’s what you need to know as you prepare your device for 510(k) submission.
Optical Device Characterization
Knowing and measuring your system’s imaging capabilities is critical. The FDA are interested in seeing clear reliable, repeatable, and accurately on specifications including:
- Focal length
- Depth of focus [field]
- Field of view
- Lateral spatial resolution
- Illumination power density
- Illumination uniformity
- Imaging system uniformity
- Signal-to-noise ratio*
- Signal-to-background ratio*
* standardized benchmarking procedures for fluorescence imaging not defined
Understanding how these specifications can impact patient outcomes must be considered and documented in your submissions.
Additionally, ANSI standards govern the safe limits of optical radiation exposure from medical devices, depending on their application. Understanding how your device’s illumination engine fits within ANSI limits of safe illumination densities will be critical for your device submission.
There are ISO and EVMA standards outlining test methods for digital still photography, which Imatest covers in detail as a freely-available resource. These standards only directly apply to color imaging applications - not fluorescence imaging. However they provide a strong starting point to understanding imaging system performance and measurement.
The American Association of Physicists in Medicine (AAPM): Task Group No. 311 - Guidance for Technical Performance Evaluation for Fluorescence-Guided Surgery Systems (TG311) has published guidance for system characterization, specific to fluorescence imaging. QUEL has adapted some of these standardized methodologies into benchmarking targets specifically for fluorescence imaging systems against spatial resolution, depth of field, fluorescence imaging uniformity, and imaging distortion.
If your methods are not clear and your data is not completely reliable, you can expect some challenging discussions to slow down your submission approval.
Device Sensitivity and Limit of Detection
Are the limits of your device’s performance adequate and clinically relevant?
Traditionally, these are difficult measurements to make in the presence of tissue optical absorption and scattering. Testing the minimum feature size and biomarker concentrations that your device can detect are critical to helping surgeons make the right calls in the surgical suite. Regulators need to know your device can do this with certainty. But how can you do it accurately?
Our concentration and depth sensitivity targets can be used to characterize fluorescence imaging systems against known fluorophore concentrations of commonly used markers in fluorescence guided surgery. If you are using a novel fluorophore, we can help develop representative targets for your product development efforts.
QUEL can also provide justifications for using our targets in place of tissue specimen and clinical-grade contrast agent for regulatory submissions.
Verification and Validation Procedures
How will you check that every device you make are functionally identical and helpful for your targeted indication? These procedures must be thoroughly documented and maintained in your QMS with clear test records.
QUEL offers shelf-stable, traceable reference standards that can serve as benchmarks for verifying fluorescence imaging system performance in V&V. Reach out to learn how our team can help provide the tools you need to ensure every device you make is the best it can be.
Predicate Device Justification
510(k) submissions assume there is an already-approved device comparable to yours. Where are they similar/different? Why is your device going to work better than what is already in use? Direct comparisons on common benchmarks are helpful but not always required.
Don’t let your product launch be slowed down by avoidable questions in regulatory approval.
QUEL Imaging helps teams navigate the 510(k) submission process to get impactful devices and biomarkers to market faster. Our expert team of biomedical optics specialists help ensure your submission addresses everything regulators want to see before they have to ask for it.
Talk to us today and see how we can help with your next submission.